Introduction:
Waldenström macroglobulinemia (WM) is a rare B-cell lymphoma that may be preceded by an asymptomatic IgM-monoclonal gammopathy of undetermined significance (MGUS). The mechanisms underlying the progression from IgM-MGUS to WM remain to be fully elucidated; however, the stepwise accumulation of genomic abnormalities has been suggested as a potential driver. Currently, the role of epigenetic regulation of DNA remains underexplored in this disease spectrum. Aberrant DNA methylation is a hallmark of oncogenesis that plays an important role in hematologic malignancies. The aim of this study was to assess differential DNA methylation in WM compared to IgM-MGUS and identify hyper- and hypomethylated pathways that may alter key cellular functions.
Methods:
This study included 15 patients with WM and 6 with IgM-MGUS. Bone marrow samples were prospectively collected and CD19+ and/or CD138+ B cells were positively selected. Subsequently, genome-wide DNA methylation analysis was performed. CpG methylation ratios were segmented into 200-bp regions and differentially methylated regions (DMRs) comparing WM to IgM-MGUS were identified. DMRs with a Q-value <0.05 and absolute methylation difference of ≥20% were considered significant and utilized for analysis. DMRs were annotated according to genomic regions of interest (promotors, 3'UTR, 5'UTR, introns, exons, CpG islands, CpG shores). Next, genes with significant promotor hyper- or hypomethylation were assessed using the Ingenuity Pathway Analysis. Pathways with a corrected p-value<0.05 were considered statistically significant and a Z-value was calculated based on the degree of differential methylation.
Results:
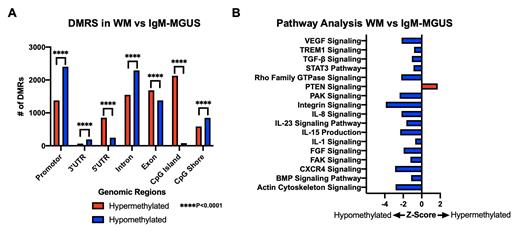
Genome-wide DNA methylation analysis revealed a significant number of DMRs (n= 15691, hyper: 8248; hypo: 7443) in WM compared to IgM-MGUS. Assessing genomic regions, promotors (hyper: 1379; hypo: 2403), introns (hyper: 1548; hypo: 2292), CpG shores (hyper: 587; hypo: 849) and 3'UTR (hyper: 66; hypo: 193) regions were observed to have a significantly greater number of hypomethylated DMRs (all comparisons, p<0.001) in WM compared to IgM-MGUS (Figure A). While exons (hyper: 1686; hypo: 1378), CpG islands (hyper: 2128; hypo: 82), and 5'UTR (hyper: 854; hypo: 246) regions had a significantly greater number of hypermethylated DMRs (all comparisons, p<0.001) (Figure A). Next, promotor methylation-based pathway analysis revealed differential methylation of multiple cellular pathways important in WM pathogenesis (Figure B). These included pathways involved in intracellular signaling and the regulation of cellular proliferation, such as the STAT3 and CXCR4 pathways which were hypomethylated, and the PTEN signaling pathway which was hypermethylated. Of interest, we have previously demonstrated the transcription factor, STAT3, to have an increased expression in WM and to be implicated in IgM secretion (Elsawa, 2011). Next, multiple cytokine signaling pathways were observed to be hypomethylated in WM compared to IgM-MGUS including members of the interleukin (IL) family (IL-1, IL-8, IL-15, IL-23), VEGF, and TGF-β. Of relevance, previous studies have demonstrated the elevation of IL-8, IL-15, and increased expression of angiogenic cytokines, including VEGF, in patients with WM (Lewicki, 2010; Anagnostopoulos, 2007). Furthermore, our group has also shown miRNA-based epigenetic regulation of IL-8 and IL-15 in WM compared to IgM-MGUS (Chohan, 2023). Lastly, several pathways critical in the regulation of the cell structure and the extracellular matrix (ECM) were found to be hypomethylated including the FGF, actin cytoskeleton, PAK, and FAK signaling pathways. Of note, the FGF/FGFR axis has been demonstrated to be dysregulated in WM, and its targeting has been shown to halt WM cell growth (Sacco, 2021).
Conclusions:
This study demonstrates differential DNA methylation of key genomic regions in WM compared to IgM-MGUS. Promotor methylation-based analysis reveals hypomethylation of several important cellular pathways involved in cytokine signaling, cell structure/ECM, and intracellular signaling. These results underscore the influence of epigenetics in the IgM-gammopathy disease spectrum and suggest the potential role of aberrant DNA methylation underlying the malignant progression from IgM-MGUS to WM.
Disclosures
Paludo:Karyopharm: Research Funding; Biofourmis: Research Funding; AbbVie: Consultancy. Wenzl:Bristol Myers Squibb: Current Employment. Ailawadhi:AbbVie, Amgen, Ascentage, BMS, Cellectar, GSK, Janssen, Pharmacyclics, Sanofi: Research Funding; Beigene, BMS, Cellectar, GSK, Janssen, Pfizer, Regeneron, Sanofi, Takeda: Consultancy. Chanan-Khan:Mayo Clinic: Current Employment; BeiGene: Consultancy, Honoraria; Ascentage: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Cellectar: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Starton: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Alpha2: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Gertz:Juno Pharmaceutics, and Sorrento Therapeutics: Other: Meetings; AbbVie: Other: Data Safety Monitoring board; Ionis/Akcea, Prothena, Sanofi, Janssen, Aptitude Healthgrants, Ashfield, Physicians Education Resource, Research to Practice, Johnson & Johnson, and Celgene: Consultancy; i3HEalth: Other: For development of educational material. Novak:Bristol Myers Squibb: Research Funding. Ansell:ADC Therapeutics: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Affirmed: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal